How Do Lead Acid Batteries Work
What are lead acid batteries and how do lead acid batteries work - a look at the technology, operation, advantages and the construction of the lead acid battery.
Home » Electronic components » this page
Battery Technology Includes:
Battery technology overview
Battery definitions & terms
Battery capacity & life
Batteries / cells in series & parallel
Zinc carbon
Alkaline cells
Zinc air cells
Lithium primary battery
NiCad
NiMH
Li-ion
Lead acid
Battery leakage cleaning, cures
Lead acid batteries are cheap, convenient and they work for many battery power applications. They are probably best known for their use in conventional internal combustion automotive vehicles where they supply the power for everything from starting, to the electronics and much more.
The lead acid battery has many advantages for automotive and many other uses: they have a large current and surge capability, which is ideal when being used to start internal combustion engines.
As a technology, lead acid batteries are well a well established technology and they can be easily manufactured with relatively low technology equipment.
However, lead acid battery technology is now being supplanted by lithium ion battery technology. These new lithium ion EV batteries are able to provide the power to drive the vehicle as well as providing the power for the electronic and electrical equipment within the car as well.
Accordingly the use of lead acid battery technology is reducing as the EV batteries are used increasingly for newer cards and other vehicles.

Lead acid battery history
The lead acid battery was the first form of rechargeable battery to be developed. The idea was originally proposed by a French Physicist named Gaston Plante in 1860.
Although another French scientist named Gautherot had discovered that platinum or silver wires that had been used to electrolyse saline water produced a current for a short duration, this was never developed into a workable battery.
The first lead acid cells were made from lead plates. The battery then had to be 'formed' by charging it so that one of the plates oxidised. The battery then increased its capacity over successive charge discharge cycles.
Plante's basic cell was later improved by a further French engineer named Faure. He managed to make the forming process much shorter by using some strips of lead oxide onto the plates. This speeded up the forming process considerably as the negative plate became lead only, and the positive plate oxidised to become lead peroxide.
Once the basic lead acid battery technology was established, the next major development involved addressing the problem that existed of water loss and the electrolyte drying up.
Batteries needed period topping up with distilled water in order to ensure their operation. This was overcome by inserting a valve into the battery. These valve-regulated lead-acid batteries, VRLA by utilising an oxygen recombination mechanism.
The gases produced inside VRLA battery were made to recombine inside and the battery was sealed for the production of batteries used for standard operating conditions.
Lead acid battery basics: how do they work
When looking at how a lead acid battery works, it is necessary to look at the basic components. The battery has comparatively few components - essentially there are four main elements:
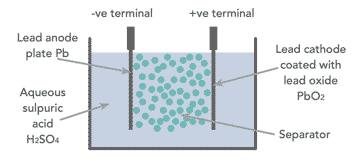
- Positive plate: This is covered with a paste of lead dioxide.
- Negative plate: This is made of sponge lead.
- Separator: This is an insulating material between the two plates, but it allows the electrolyte and the ions into it to enable conduction without the two plates touching.
- Electrolyte: This consists of water and sulphuric acid
These constituents are all contained within a plastic container which acts to keep the electrolyte in and the battery together.
Although the plates or electrodes of the lead acid battery are designated as being lead, lead itself is too soft to be used on its own. Accordingly small quantities of other metals are added to the lead to provide the additional strength required. They can also improve the electric performance. Typical additive metals include: antimony, calcium, tin and selenium.
The overall battery will normally consist of several cells placed in series to give the required voltage as each cell is capable of providing an EMF of 2.1 volts.
Charging lead acid batteries
In order to enable the basic lead acid cell to produce a voltage, it must first receive charge. The voltage applied to provide this must be greater than the 2.1 volts to enable current to flow into the cell. If it were less than this, charge would actually flow out of it.
Lead acid batteries require a constant current constant voltage charge form of charging. To achieve this, a regulated current raises the terminal voltage on the battery or cell until the upper charge voltage limit is reached. At this point the current drops due to saturation.
Charge times for giving a battery a complete charge are typically around twelve to eighteen hours for most common forms of battery but they can be up to 36 or even 48 hours for large batteries that might be used for temporary power, etc.
It is possible to provide a fast charge by utilising a more complex form of charging and this will entail a more complex charger, which in trun will cost more. This might be a cost effective approach for some situations. Here higher currents and multi-stage charging enable the battery not to be unduly stressed while reducing charge times.e methods, the charge time can be reduced to eight to ten hours, but this does not give a full charge.
Although the lead acid battery charging works well for many applications, when compared to other newer technologies like lithium ion battery technology, they are relatively slow.
Once charged, the cell or battery will be able to provide charge to external circuits, often operating over several hours dependent upon the drain on the cell or battery.
Guidelines and tips for maintenance of lead acid batteries
There are a few tips and guidelines to get the most out of a lead battery by charging it correctly. This will ensure safety, best operation, and longest life of the battery.
Charge in well ventilated area: During the charging process, hydrogen gas can be created and if confined in a small space, this could be explosive.
Don't store batteries in state of low charge: In order to prevent a process called sulphation, batteries should not be stored in a state of low charge - ideally they should always be charged after use.
Avoid "flattening" the battery: Totally dischraging or flattening a battery can significantly shorten its life. It is often found that once a car battery has been totally flattened, then its capacity is significantly reduced and it is likely to become totally discharged again. Old batteries that become flat should be replaced to avoid additional issues.
Ensure battery plates are covered: In some batteries it is necessary to top up the electrolyte level to ensure the plates are covered. This should be done using distilled water (not ordinary tap water as this contains contaminants that will redice the effectiveness of the electrolyte). Also never add new electrolyte. Top-ups are not required for many modern low maintenance types and it is not possible to gaina access to the battery chambers to do this.
Do not over-fill: When filling only fill to the designated level. Overfilling can cause the acid to spill out during charging.
Gas bubbles on the plates indicate that battery is virtually fully charged: When gas bubbles start to appear ont he plates of what are called flooded lead acid batteries, this indicates the battery is fully charged and it starts to split the water molecules into hydrogen (negative plate) and oxygen (positive plate).
Avoid charging at high temperatures: If the temperature is above 49 or 50°C, then avoid charging.
Do not allow the battery to freeze: Never allow a lead acid battery to freeze as this can cause irreparable mechanical damage. It is worth noting that a fully charged battery freezes at a lower temperature than a partially charged one.
Lead acid battery self discharge
The self discharge characteristic of the lead acid battery is relatively good. At a room temperature of 20°C the self-discharge rate is around 3% per month.
In theory a lead acid battery can be stored for up to 12 months without recharge. However at higher temperatures the self discharge is higher. At 30°C the self-discharge increases and a recharge will be needed after 6 months. Letting the battery drop below 60% for some time causes sulphation.
Sulphation is a process which reduces the capacity of lead acid batteries. During normal use, small sulphate crystals form, but these are normal and are not harmful. During prolonged charge deprivation, however, the amorphous lead sulphate converts to a stable crystalline and deposits on the negative plates. This leads to the development of large crystals that reduce the active material within the cell and result in a reduction of capacity within the cell.
Lead acid battery advantages & disadvantages
Although lead acid batteries are widely used because they have a number of distinct advantages, they also have several major disadvantages. All these need to be considered when deciding upon whether to use this technology or not.
Lead Acid Battery Advantages
- Mature technology
- Relatively cheap to manufacture and buy (they provide the lowest cost per unit capacity for rechargeable cells)
- Large current capability
- Can be made for a variety of applications
- Tolerant to abuse
- Tolerant of overcharging
- Wide range of sizes and specifications available
- Many producers worldwide
Lead Acid Battery Disadvantages
- Fails after a few years use lifespan typically 300 - 500 cycles
- Cannot always be used in a variety of orientations
- Corrosive electrolyte (can cause burns to people and corrosion on metalwork)
- Lead is not environmentally friendly
- Acid needs disposing of with care
- Not suitable for fast charging
- Must be stored in charged state once electrolyte introduced
- Typical charging efficiency only around 70%
Like all battery technologies and battery systems, lead acid batteries have their advantages and disadvantages. The advantages and disadvantages need to be considered when looking at a battery technology for a new development. For existing systems, these need to be considered when handling, using and maintaining them.
The lead acid battery is very well established. It has been in use for over 150 years and has been one of the mainstays of the automotive industry.
The lead acid battery has a high current capability, low cost and it is tolerance to abuse. This makes the lead acid battery ideal for many applications.
However with the move to more environmentally friendly sources of power, electric vehicles now appear to be the future with manufacturers and legislation pointing to the phasing out of the internal combustion engine. For electric vehicles Lithium Ion technology provides better performance, they are more environmentally acceptable and they have the performance to enable electric vehicles to succeed. As such the lead acid battery is likely to be considerably less widely used.
 Written by Ian Poole .
Written by Ian Poole .
Experienced electronics engineer and author.
More Electronic Components:
Batteries
Capacitors
Connectors
ADC
DAC
Diodes
FET
Inductors
Memory types
Phototransistor
Quartz crystals
Relays
Resistors
RF connectors
Switches
Surface mount technology
Thyristor
Transformers
Transistor
Unijunction
Valves / Tubes
Return to Components menu . . .