Battery Types & Technologies: a primer
There are various different technologies for electrical batteries and cells both rechargeable and non-rechargeable and understanding how they work can enable them to be used more effectively.
Home » Electronic components » this page
Battery Technology Includes:
Battery technology overview
Battery definitions & terms
Battery capacity & life
Batteries / cells in series & parallel
Zinc carbon
Alkaline cells
Zinc air cells
Lithium primary battery
NiCad
NiMH
Li-ion
Lead acid
Battery leakage cleaning, cures
Batteries are becoming more widely used. As the use of portable and mobile equipment increases, so does the use of battery technology. There are many different types of battery or cell and these use different technologies.
The increasing demands being placed on batteries has meant that the technology has developed considerably in the past few years, and more development can be expected in the future.
With the huge demand for batteries, there is a wide variety of different types of battery and cell technologies available. These range from the established non-rechargeable technologies such as zinc-carbon and alkaline batteries to rechargeable batteries that have moved from NiCd through NiMH cells to the newer lithium ion rechargeable batteries.

With a huge need for batteries, there is a large amount of battery technology development underway and new types of betteries and cells will no doubt become available offering even higher levels of performance.
Another area of battery technology that is becoming more important is the green or environmental aspects. Some of the old battery technologies contain chemicals which can be considered as toxic.
Now new designs are seeking to use more environmentally friendly chemicals. Nickel cadmium cells are now considered as being environmentally unfriendly and are not as widely used as they were previously.
Other types of battery also contain harmful chemicals and this is likely to have a significant impact on the direction of future developments.
Basic battery and cell concepts
Looking at the very basics of battery technology, a battery is a combination of two or more electrochemical cells. These electrochemical cells store energy in the form of chemical energy, and this is converted into electrical energy when connected to an electrical circuit in which an electrical current can flow.
All the different types of battery or cell consists of two electrodes with an electrolyte placed between them. The negative electrode is known as the cathode, while the positive electrode is known as the anode. The electrolyte between them can either be a liquid or a solid.
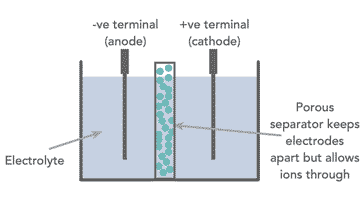
Today many cells are enclosed in a special container, and there is an element known as a separator placed between the anode and cathode. This is porous to the electrolyte and prevents the tow electrodes from coming into contact with each other.
The potential difference across the terminals of the battery is known as the terminal voltage. If the battery is not passing any current, e.g. when it is not connected to any circuit, then the terminal voltage seen is the open circuit voltage and this equals the EMF or electro-motive force of the battery.
It is found that all batteries have a certain level of internal resistance. As a result the terminal voltage falls when it is connected to an external load. As the battery becomes exhausted it is found that the internal resistance rises and the voltage under load falls.
Types of battery: primary and secondary cells
Although there are many different types of battery, there are two main categories of cell or battery that can be used to provide electrical power. Each type has its own advantages and disadvantages and therefore each type of battery is used in different applications, although they can often be interchanged:
- Primary batteries: Primary batteries are essentially batteries that cannot be recharged. They irreversibly transform chemical energy to electrical energy. When the chemicals within the battery have all reacted to produce electrical energy and they are exhausted, the battery or cell cannot be readily restored by electrical means.
- Secondary batteries: Secondary batteries or secondary cells are different to primary ones in that they can be recharged. The chemical reactions within the cell or battery can be reversed by supplying electrical energy to the cell, restoring their original composition.
In many respects there has been a major move to use rechargeable batteries and cells. The reason for this is that recharging cells and batteries is far more cost effective and also does not batteries to be replaced every time they run out of charge.
Early types of rechargeable like the lead acid battery used in many automobiles these days were not suitable for small electrical and electronic gadgets. Instead Nickel cadmium batteries were first used and then these migrated to Nickel Metal Hydride battery technology as this has a much lower environmental impact.
Now lithium ion battery technology is being widely used for everything from many electronic gadgets and smartphones and laptops through to electric vehicles.
The pace of development has been huge and more is expected.
Different types of standard battery and cell sizes
It is essential that batteries, and in particular primary batteries can be changed when their useful life is over. As a result batteries normally come in standard battery sizes so that batteries from different manufacturers can be used. As a result there are a number of standard battery sizes that are used.
A summary of the more common standard battery sizes is given below:
| Standard Cell & Battery Sizes | ||
|---|---|---|
| Cell type | Diameter mm |
Height mm |
| AAA | 10.5 | 44.5 |
| AA | 14.5 | 50.5 |
| C | 26.2 | 50.0 |
| D | 34.2 | 61.5 |
Electrical cell or battery types
There are many different types of battery or cell technology that are available. Each different type of battery technology has its own advantages and disadvantages.
Accordingly different types of cell or battery technology may be used in different applications. The table below gives a summary of some of the different types that are in more common use today.
| Battery Types & Their Proeprties | |||
|---|---|---|---|
| Cell or battery type | Nominal voltage V |
Characteristics | |
| Types of primary cells and batteries | |||
| Alkaline manganese dioxide | 1.5 | Widely available, providing high capacity. Shelf life normally up to about five years. Capable of providing moderate current. Read more | |
| Lithium thionyl chloride | 3.6 | Good for low to medium currents. High energy density and long shelf life. | |
| Lithium manganese dioxide | 3.0 | Long shelf life combined with high energy density and moderate current capability. | |
| Mercury oxide | 1.35 | Used for button cells but are phased out now because of the mercury they contain. | |
| Silve oxide | 1.5 | Good energy density. Mainly used for button cells. | |
| Zinc carbon | 1.5 | Widely used for consumer applications. Low cost, moderate capacity. Operate best under intermittent use conditions. Read more | |
| Zinc air | 1.4 | Mostly used for button cells. Have a limited life once opened and low current capability but a high energy density. New types being developed for high current use, even in electric vehicles. Read more | |
| Types of secondary cells and batteries | |||
| Nickel cadmium NiCd |
1.2 | Were in very common use, but now giving way to NiMH cells and batteries in view of environmental impacts. Low internal resistance and can supply large currents. Long life if used with care. Read more | |
| Nickel metal hydride NiMH |
1.2 | Higher capacity but more expensive than NiCads. Charging must be carefully controlled. Being used in many applications where NiCads were previously used. Read more | |
| Lithium ion Lion |
Highest capacity and they are now widely used in many laptops, mobile phones, cameras . . etc. Charging must be carefully controlled and often have a limited life ~ typically 300 charge discharge cycles. Read more | ||
| Lead acid | 2.0 | Widely used for automotive applications. Relatively cheap, but life expectancy often short. Read more | |
Battery management systems
One area where battery technology has developed in leaps and bounds is with the use of battery management systems. Early rechargeable batteries like elad acid and Nickel Cadmium batteries used little or nothing in the way of battery management.
Nowadays, modern rechargeable cells and batteries like those using lithium ion technology often have the electronics for battery management systems included within them.
These battery management systems monitor the state of the battery, understanding the level of charge available, the capacity of the battery (it typically reduces with usage for battery technologies like lithium ion), temperature and so forth.
The battery management systems control the usage of the battery, cutting off the charge when the battery reaches the required charge level, stopping it becoming totally exhausted which can cause damage, indicating the level of charge available, and geenrally managing the operation of the battery.
Essentially the battery management system ensures that the optimum operation of the cell or battery is obtained, preventing aspects such as under-charge, over-charge or delivering too much current, or being charged too fast.
The performance of battery technology has improved considerably in recent years. As the demands being required of batteries has increased with greater capacity being required in smaller spaces and greater levels of reliability, so considerable amounts of research have been invested in trying to meet the new requirements.
The research has resulted in much longer times between charge, higher capacity levels and greater degrees of reliability. For the future, the demands being placed on batteries will only increase, and no doubt the technology will improve beyond all measure.
 Written by Ian Poole .
Written by Ian Poole .
Experienced electronics engineer and author.
More Electronic Components:
Batteries
Capacitors
Connectors
ADC
DAC
Diodes
FET
Inductors
Memory types
Phototransistor
Quartz crystals
Relays
Resistors
RF connectors
Switches
Surface mount technology
Thyristor
Transformers
Transistor
Unijunction
Valves / Tubes
Return to Components menu . . .


