Zinc Carbon Battery & Cell
Zinc carbon batteries and cells are a cheap form of non-rechargeable battery used to power a variety of electrical and electronic devices.
Home » Electronic components » this page
Battery Technology Includes:
Battery technology overview
Battery definitions & terms
Battery capacity & life
Batteries / cells in series & parallel
Zinc carbon
Alkaline cells
Zinc air cells
Lithium primary battery
NiCad
NiMH
Li-ion
Lead acid
Battery leakage cleaning, cures
The zinc-carbon cells and batteries are non-rechargeable, i.e. primary cells and they are one of the oldest form of batteries in current use, and they deliver around 1.5 volts per cell similar to alkaline manganese cells
These batteries or dry cells as they have been called have been known for over 140 years and they were the first dry cells to be developed and they have their basis in the old wet Leclanche cells of the 1800s that used a wet electrolyte.
In general, the zinc–carbon type of cell or battery has the attributes of low cost, ready availability, and acceptable performance for many applications. These are particularly important for their use in emerging third world countries, although their use in the USA and Europe is declining as alkaline (alkaline manganese dioxide) cells and batteries increases.

How zinc carbon cells were developed - history
The modern zinc carbon cell can trace its ancestry back to the wet Leclance cell. This was developed in 1866 by Georges Leclanche, and it consisted of a zinc anode and a manganese dioxide cathode which was wrapped in a porous material. This was then dipped in a jar of ammonium chloride solution as the electrolyte.
Being what was termed a wet battery or cell, this was not suitable for many uses as great care needed to be taken to ensure the electrolyte did not spill.
The next major development was to make a dry version of the cell. This fell to Carl Gassner, a German physician, scientist and inventor. In 1886 he developed a cell using a casing made of zinc sheet metal as the anode and a paste of plaster of Paris. This prevented the use of a wet electrolyte and enabled the cells to be used in many more situations.
Then, 1898 Conrad Hubert first used the new dry cells to power a portable light. Later he and the manufacturer of the cells formed a new company which they called the Eveready Battery Company. This was a major name in batteries for very many years afterwards.
Right from the earliest versions of the Leclanché cell, it was commercially successful because the zinc for the anode, the manganese dioxide for the cathode(which occurred naturally), and ammonium chloride salt for the electrolyte were available and inexpensive.
During the twentieth century very many improvements were made to the standard zinc carbon dry cells and a huge number were manufactured.
Aspects including the introduction of stable but thin separators, improved seals, and the use of ammonium chloride as the electrolyte, all gave much better high drain performance.
Also the technology improved to give cells and batteries that leaked less - there was even a range that were designated leakproof, although whether this would be allowed by today's trading standards remains open to debate.
The usage of the zinc carbon cell started to fall as the alkaline cells (alkaline manganese dioxide cells) were introduced and their usage started to take over, especially in Europe and the USA.
Comparison with other forms of battery and cell
The zinc carbon cell produces a terminal voltage of around 1.5 volts when it is new and when it is not loaded.
Being a primary cell, it is not rechargeable and once the charge is spent, it needs to be disposed.
It is interchangeable with the alkaline manganese diode batteries and cells that are becoming more popular. The alkaline batteries and cells offer a better level of performance, both in overall charge capacity and hence their lifetime as well as the current they can deliver.
They can often be used interchangeably with some rechargeable batteries and cells. nickel cadmium, NiCd cells were popular many years ago and and provide an output voltage of around 1.25 volts. Accordingly these were made in similar sizes to zinc carbon batteries and were used interchangeably. Nowadays the environmental aspects of nickel cadmium batteries and cells mean they are no longer used.
NiCds were replaced by nickel metal hydride, NiMH cells. These only have a voltage output of around 1.1 volts, but can still be used interchangeably for most electrical and electronic gadgets where the same size batteries and cells are available.
One area where there is a large change between the zinc carbon and other cells and batteries is where lithium based cells are used. Not only are there the lithium based primary or on-rechargeable cells, but also the lithium ion family of rechargeable cells and batteries.
The lithium primary cells have a voltage of three volts and this means that they have double the voltage of the zinc carbon and therefore they cannot be directly replaced. Normally different sized batteries may be used to prevent accidental interchanges that could be disastrous.
Also the lithium ion rechargeable batteries have an output voltage of around 3.3 to 3.9 volts. These batteries have a high charge density and can provide high performance. Overall their performance is much higher than the zinc carbon cells.
Zinc carbon battery advantages & disadvantages
Having looked at the way zinc carbon cells compare with other forms of cel and battery, t is worth summarising their advantages and disadvantages.
Like all forms of battery, zinc carbon cells and batteries have their own set of advantages and disadvantages. These need to be weighed up when considering to use them or another battery technology like alkaline batteries or even lithium ion batteries.
Zinc carbon batteries and cells advantages
Cost: One of the advantages is that zinc carbon batteries and cells are made from chemicals that are freely available at low cost.
The chemicals required for zinc carbon batteries and cells are common and they themselves manufactured in many countries and there are no restrictions on them.
Ease of manufacture: The processes behind the manufacture of these batteries and cells are very well established and manufacturing costs are low.
With manufacturing techniques well established and widespread, these cells and batteries can be manufactured in virtually all countries and for very little cost.
Availability: As zinc carbon batteries and cells have been manufactured for very many years, they are available in very many sizes and packages.
Zinc carbon batteries and cells are probably available in more standard sizes than any other battery technology as they have been used for many different applications over the years.
Zinc carbon batteries and cells disadvantages
Capacity: The capacity of these batteries and cells is not nearly as high as that of even alkaline manganese dioxide batteries and cells. Alkaline cells can offer up to eight times the charge / lifetime of zinc carbon, especially when used under heavy or high current conditions.
Current capability: The current capability is low when compared to alkaline cells and many rechargeable battery types.
Leakage: One of the key issues with these cells and batteries was that when left in equipment for any time, the caustic chemicals would react with the case and leak out. This caustic leakage often caused damage to equipment. Accordingly the cells needed to be replaced
Caustic chemicals: The chemical used within zinc carbon cells can be caustic, although not as harmful as some used in other types of cell, which have been discontinued now.
Non-rechargeable: Zinc carbon technology is not rechargeable like the popular alkaline manganese dioxide cells.
These are some of the main advantages and disadvantages of zinc carbon batteries and cells. It is always worth investigating fully the characteristics of the technology to discover all that needs to be known for any specific application or situation.
Construction of a zinc carbon battery cell
The construction of the zinc-carbon batteries and cells is relatively straightforward.

Note: beware if doing this that some chemicals are corrosive and can cause irritation, etc
Many zinc carbon battery cells are in the form of cylinders and the construction of these may be a little different to those of the PP3 style 9V battery made from several individual cells.
For many zinc carbon cells the container is a zinc can which forms the anode.
The bottom and sides of the can contain a paper separator layer which is impregnated with ammonium chloride, NH4Cl) along with a thickening agent to form an aqueous electrolyte paste.
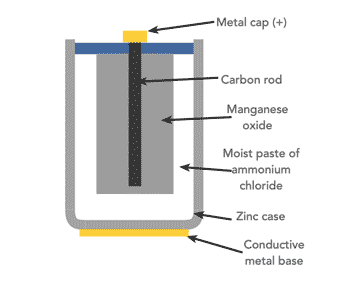
The paper separator prevents a short circuit from forming by protecting the zinc can from making contact with the cathode, which is a mixture of powdered carbon usually in the form of graphite powder and manganese dioxide, MnO2, which is packed around a carbon rod.
Carbon is the only practical conductor material because every common metal quickly corrodes in the positive electrode when in the presence of a salt-based electrolyte.
Zin carbon chemistry
There are several chemical reactions that occur within a zinc carbon cell. It is an electrochemical device, converting chemical energy into electrical energy. Reactions take place at different points within the cell.
The overall reaction can be summarised by the following chemical equation.
The zinc carbon better and cell technology is still widely used across the globe. Although it is giving way to alkaline cells and batteries which perform better, providing a higher capacity and current capability, zinc carbon is still used because of the much lower cost.
Zinc carbon battery and cell technology has been in used for over 140 years and its use will continue, although this is falling, especially within the USA and Europe.
 Written by Ian Poole .
Written by Ian Poole .
Experienced electronics engineer and author.
More Electronic Components:
Batteries
Capacitors
Connectors
ADC
DAC
Diodes
FET
Inductors
Memory types
Phototransistor
Quartz crystals
Relays
Resistors
RF connectors
Switches
Surface mount technology
Thyristor
Transformers
Transistor
Unijunction
Valves / Tubes
Return to Components menu . . .


